Protection of Honey Combs From Wax Moth Damage
Protection of Honey Combs From Wax Moth Damage
American Bee Journal, August, 1999
by JEAN-DANIEL CHARRIERE and ANTON IMDORF
Swiss Federal Dairy Research Station
(FAM)
Bee Department, Liebefeld
3003 Bern, Switzerland
Translation by Ro. Raynor
The following moths are regarded as pests of bee products:
Class: Insects – Insecta
Order: Butterflies – Lepidoptera
Family: Pyralids – Pyralidae
Species:
Greater Wax Moth – Galleria mellonella L.
Lesser Wax Moth – Achroia grisella
Fruit (pollen) Moth – Vitula edmansae
Mediterranean Flour Moth – Esphestia kuehniellaOf all moths, the Greater Wax Moth causes the greatest damage in apiaries which lead to material and financial losses every year. For this reason, we propose to study only the biology of the Greater Wax Moth more closely. The methods employed in combating Galleria mellonella are generally effective against other moths identified as pests of bee products.
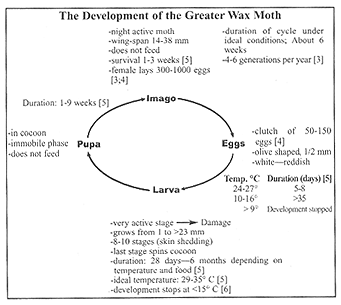
Biology of the Greater Wax Motha) Geographical distribution
The geographical distribution corresponds reasonably with that of the bee. Distribution is limited by the inability of the pest to withstand prolonged periods of cold. This explains why Wax Moth problems are less acute in higher altitude locations or do not occur at all [1].
b) Pathology
Adult Wax Moths cause no damage because their mouthparts are atrophied. They do not feed during their adult life. Only larvae feed and destroy combs. However, adult Wax Moths and larvae can transfer pathogens of serious bee diseases (e.g. foulbrood). In colonies infested with foulbrood. the feces of Wax Moths contain large amounts of Paenibacillus larvae spores [2].
c) Development stages
Galleria development goes through 3 consecutive stages–egg, larva and pupa. This sequence is only interrupted if the temperature is too low or when there is no food. Therefore, the cycle can last between 6 weeks and 6 months depending on temperature and food. According to the literature, over-wintering can take place as egg, larva or pupa.
d) The egg
Normally, females lay their eggs by means of their ovipositor into crevasses and gaps. This puts them out of reach of the bees and prevents their destruction.
e) The larva
After hatching, the young larva immediately searches for a comb in order to feed and to build the silk-lined feeding tunnels. Speed of growth is directly dependent on temperature and food supply. Under ideal conditions the larval weight can double daily during the first 10 days [4]. The metabolic warmth, which is created by this rapid growth, can increase the temperature in the spun silk nests far beyond the environmental temperature. The larva feed in particular on impurities occurring in wax, such as feces and the cocoons of bee larvae as well as pollen. The larva also eat wax. Larvae, which have been reared exclusively on pure wax (foundation, fresh comb), do not complete their development [4; 13]. Dark, old combs that contain many bee larval cocoons are most at risk. At the end of the larval stage, the larva spins a very resistant silk cocoon on a firm support, such as wooden frames, hive walls or in the comb storage chest. Frequently the larva spins its cocoon in a hollow it had bored into the wood.
f) The pupa
In the cocoon, the larva changes into a pupa and then into the adult moth. These metamorphoses last from one to 9 weeks.
g) The adult Insect (imago)
Size and color of the imago vary considerably, depending on food composition at the larval stage and on the duration of the various developmental stages. Females are larger than males [5]. The females start laying eggs between day 4 and 10 after emergence from the cocoon [5]. At dusk, the females attempt to enter the beehive to lay their eggs. If the colony is strong enough to repel the wax moth, they lay their eggs outside in cracks in the wood.
Possibilities for controlling Wax Moth
In beehives:
-Allow only strong colonies in an apiary. (The bee itself is the most dangerous enemy of the Wax Moth).
-Never leave comb or wax in an unoccupied hive.
-Periodically clean your Varroa inserts.
-Replace combs regularly.
-After mass invasion of Wax Moths, destroy their eggs on combs, frames and hives (e.g. sulphur vapor).
In comb storage chests: (see table)
Main rule: For all control strategies, it is necessary to inspect stored material regularly during the warm season.
- Technical methods
- Physical methods
- Biological methods
–Bacillus thuringiensis spores
The bacterium Bacillus thuringiensis was discovered in 1911 and has been successfully used for plant protection for several years. The bacterial strain of the product B-401 was selected in particular for its activity against the Wax Moth. The bacterium produces spores containing a toxin. When the larvae ingest the spores, the toxin is freed and damages the intestinal walls. This results in the death of the larvae. Adult Wax Moths do not feed and are therefore not endangered by this product. B-401 is harmless for vertebrates (man, livestock) and bees, and leaves no residues in wax or honey. (It is not currently available for sale to U.S. beekeepers.)
- Chemical methods
-Sulphur (sulphur dioxide, SO2)
Burning of sulphur strips or spraying of SO2 from a pressurized vessel are the two main control methods using sulphur. This is still one of the most effective means against Wax Moths. It is highly volatile, not fat-soluble and therefore poses only a slight danger to bees, wax, and honey. After removing comb from the colonies, it is advisable to wait one or two weeks before treatment (SO2 is ineffective against eggs). For more safety, the treatment can be repeated after 2 weeks.
-Acetic acid
Acetic acid vapor instantly kills eggs and moths. The larva, especially in the cocoon, is more resistant and must be exposed to the vapors for longer [3]. For this reason, the combs must be treated immediately after removal from the colonies, before eggs can develop into larvae.
-Formic acid
Professional beekeepers in Europe successfully use formic acid against Wax Moths. The effects are comparable to that of acetic acid.
-Paradichiorobeuzene (PDCB)
In high concentrations, PDCB can be toxic to bees. If several combs are put directly into the colony from a storage chest without airing, heavy damage may occur and can result in the death of the colony.
| Control Possibilities Against Wax Moths In Stored Combs | |||
| Method | Advantages(+) Disadvantages (-) |
Procedure/Remarks | |
| Technical | + no residues | ||
| – | – Sorting comb | – supplementary measure – separate dangerous old comb from foundation and new comb |
|
| – immediately melt old wax | – supplementary measure | ||
| – storage in a cool, light, and airy place | + simple | – Moths fear light and drafts; e.g. shed, porch; – Protect against weather, rodents and insects |
|
| Physical | + no residues | ||
| – cool storage (<15ºC) | + effective- infrastructure, long term method | – cellar, cool place – good air circulation in comb stack |
|
| – frost treatment | + effective + kills all stages – expensive infrastructure |
– 2 hours at -15ºC or 3 hours at -12C or 4.5 hours at -7ºC [5] – strict period of frost |
|
| – heat treatment | + effective + kills all stages – infastructure (warm air blower) – risk of wax melting |
– 80 minutes at 46ºC or 40 minutes at 49ºC- good air circulation – accurate temperature control |
|
| Biological | – spores of Bacillus thuringiensis (B-401) | + no residues+ long-term effect (2-3 months) – average effect against the Lesser wax moth – expensive |
– observe instructions – ensure good distribution on the combs – observe sell-by-date and storage conditions (living organisms) – if combs already infested, 1 x sulphur, then B-401 – ideal for the beekeeper with a few colonies |
| Chemical | – Sulphur | + effective + good pollen consevation against molds – regular repeats – ineffective against eggs – fire danger |
– treatment from above (SO2 heavier than air) – do not breath in vapors (respiratory and eye irritant) – burn in a small sulphur stove – treat every four weeks (in summer) – 1 strip per 100 liters (about 3 supers) – SO2 in spray can – 1 second (=2.5g SO2) per honey super or – 3-4 seconds per 100 liters hive volume – no fire danger |
| – Acetic Acid | + effective + no problem residues + kills all stages + kills Nosema spores [10] – attacks metal parts – regular repeats – caution when handling |
– treatment from above (vapors heavier than air) – do not breath in vapors, avoid contact with skin – 200ml acetic acid (60-80%) per 100 liters per hive volume [6;7;10;11] – in summer, treatment repeated 1-2 times with an interval of 2 weeks |
|
| – Formic Acid | + effective + no problem residues + kills all stages – attacks metal parts – regular repeats – caution when handling |
– treatment from above – do not breath in vapors, avoid contact with skin – 80ml formic acid (85%) per 100 liters hive volume [12] – in summer, treatment repeated 1-2 times with an interval of 2 weeks |
|
| – Paradichlorobenzine (PDCB) |
+ simple handling + effective – residues in wax and honey!!! – ineffective against eggs – toxic to bees at high dosages |
-use cannot be recommended – aerate combs for 2-3 days before inserting into colony – treatment from above |
|
Contamination of wax and honey by paradichiorobenzene (PDCB)
PDCB is a highly volatile and lipophilic (easily soluble in fat and wax) substance. Beeswax can take up this material and a part of it may later migrate into honey. Honey analyses from Germany and Austria show that PDCB residues in honey are not rare. This applies to native as well as imported honeys.
Even when measured values pose no problems as far as human toxicology is concerned (an experiment on carcinogenic effects is ongoing), the reputation of honey as one of the last natural products may be damaged in the eyes of the public. Therefore, all beekeepers who are concerned about the quality of bee products are advised not to use PDCB and it is recommended that alternative control strategies be employed.
German experiments (K. Wallner, Hohenheim, 1992) [8]
-PDCB – Residues in honey
| 109 analyzed German honey samples 51 honeys tainted with PDCB |
|
| ug/kg | Samples |
| 3-5 | 29 |
| 6-10 | 16 |
| 11-20 | 3 |
| 21-50 | 3 |
| >50 | 0 |
(limit of detection at 3 micrograms per kilogram honey)
1 ug/kg corresponds with 1 millionth of a gram in 1 kilogram honey.
-Paradichlorobenzene in wax
The amount of PDCB stored in wax depends on the duration of exposure and the wax surface area. Foundation takes up PDCB more quickly than wax as a block (table 1). Wax takes up PDCB like a sponge. The more PDCB crystals are added to combs and the longer PDCB acts on the combs, the higher the substance stored in the wax.
Table 1: Uptake capacity of a 1kg wax block
| Time Span | Paradichlorobenzene |
| After 1 Month | 27.3g |
| After 2.5 Months | 38.5g |
| After 9 Months | 83.5g |
Evaporation of PDCB from beeswax.
-Airing
Airing of combs over 1-2 days before insertion into the colony avoids visible damage to bees. Despite this, considerable amounts of PDCB may still be present in wax. Airing over several weeks is not enough to remove PDCB from wax completely (fig.2).


The amount and speed of removal are above all temperature-dependent. Thus, the considerably higher temperature in the colony causes PDCB evaporation from combs not previously aired enough. If these cells are now filled with honey, PDCB migrates slowly into the honey.
-Melting old wax
When old comb is melted, the residues persist in the new wax. Examinations of wax carried out here have shown that the majority of commercial wax in Switzerland contains PDCB residues of 5-10 mg/kg.
Stability of PDCB In honey
-PDCB evaporates reluctantly from honey and only from the topmost layer.
-Honey cannot be aired as long as needed, since it attracts water and odors.
-There is no possibility of significantly reducing paradichlorobenzene content of honey later.
-Residues of PDCB in honey are not permitted in Switzerland. Honeys with residues are rejected by the Cantonal chemists. Honeys with any residue that is not normal will be rejected by British packers.
Bibliography:
[1]Jeanne F., 1982, Principaux papillons parasites de la cire et moyens de lutte. Bul. tech. apic.,9(2), 85 – 92 [Principal moth parasites in wax and means of control.]
[2]Borchert A., 1966, Die Krankheiten und Schadlinge der Honigbiene. Hirzel Verlag Leipzig [Diseases and pests of the honey bee]
[3]Moosbeckhofer R., 1993, Wachsmotteneine Gefahr fur den Wabenvorrat. Bienenvater, 6, 261 – 270 [Wax moths-a danger for stored wax comb.]
[4]Morse R.A., 1978, Honey bee pests, predators and diseases. Cornell University Press
[5]Shimanuki H., 1981, Controlling the greater wax moth. USDA publication
[6]Ritter W., Perschil F., Vogel R., 1992, Vergleich der Wirkung verschiedener Methoden zur Bekampfung von Wachsmotten. ADIZ (1), 11 – 13 [Comparison of the effect of various methods for combatting wax moths.]
[7]Mautz D., 1990, >>Giftiger Honig<<, lmkerfreund (11), 12 – 14 [„Poisonous honey“]
[8]Wallner K., 1991, Das Verhalten von Paradichlorbenzol in Wachs und Honig ADIZ (9), 29 – 31 [The behavior of PDCB in wax and honey.]
[9]Spurgin A., 1991, Wachsmottenbekampfung. ADIZ (9), 25 – 26 [Controlling wax moth.]
[10]Jordan R., 1957, Essigsaure zur Bekampfung der Wachsmotte und vor allem aber zum Entkeimen nosemainfizierter Waben. Bienenvater, 78 (6), 163 – 169 [Acetic acid for controlling wax moth and in particular for disinfecting nosema-infected combs.]
[11]Gerig L., 1985, Der Schweizerische Bienenvater, Veriag Sauerlander, 16. Aufl.
[12]Krasnik M., personliche Mitteilung [personal communication.]
[13]Altermatt F., 1996, Die grosse Wachsmotte, eine Uberlebensspezialistin?, Selbstandige Arbeit, Gymnasium Laufental [The greater wax moth, a survival specialist? Independent work, Laufental Grammar School.]


